The research group led by Prof. Sheng Ye from Life Sciences Institute (LSI), Zhejiang University has recently published a paper entitled “FtsZprotofilaments use a hinge-opening mechanism for constrictive force generation” in Science on July 26, 2013.
FtsZ is a guanosine triphosphatase (GTPase) that polymerizes into protofilaments at the bacterial division site. These protofilaments further organize to a structure termed the Z ring. Z ring recruits the accessory division proteins to the septum and also provides mechanical forces needed to constrict the membrane and reduce the cell width. However, how Z ring generates mechanical force is unclear. While one popular model suggests that mechanical forces are generated by means of a change in FtsZ structure induced by guanosine triphosphate (GTP) hydrolysis, nucleotide-dependent conformational transitions have yet to be observed in FtsZ monomer structures. Such transitions may be a feature of FtsZ only in its native protofilament-forming state. Researchers from Zhejiang University of China sought to resolve this question by obtaining high-resolution structures of guanosine diphosphate (GDP)–bound Mycobacterium tuberculosis FtsZ (MtbFtsZ) filaments. The results suggest a complex and dynamic FtsZ protofilament network with a high degree of plasticity that is capable of generating forces to drive cytokinesis, during cycles of hydrolysis, while maintaining the structural integrity of individual monomers.
The structure highlights a hydrolysis-dependent conformational switch at the T3 loop that leads to longitudinal bending between subunits, which can generate sufficient force to drive cytokinesis. More specifically, GTP γ-phosphate stabilizes the T3 loop conformation in a compact state (tension or T state) (Figure 1) This T-state conformation is necessary for the longitudinal assembly of a straight FtsZ protofilament, in which the T3 loop interacts extensively with the T7 loop of the top subunit. In contrast, in the GDP-bound MtbFtsZ protofilament structure, the T3 loop adopts a relaxed conformation (R state) in the absence of the -phosphate, indicating that it is flexible and can be in either state. Because both β- and γ- phosphates are negatively charged, the release of γ-phosphates by hydrolysis may trigger the T3 loop conformational transition from the T state to R state, driving the hinge-opening event around the pivot point (L269 in MtbFtsZ). Additionally, FtsZ contains a flexible C-terminus that binds FtsZ, which is attached to the membrane through its C-terminal amphipathic helix. Thus, in the context of FtsZ division function, the straight-to-curved transition can exert constrictive forces on the envelope (Figure 2).
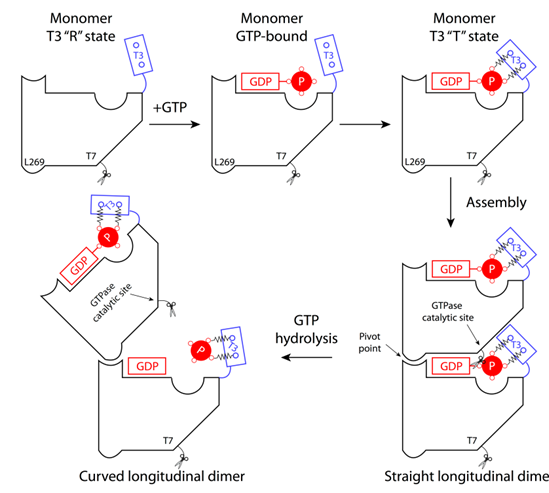

Figure 1. hydrolysis-mediated conformational switch Figure 2. Schematic of Membrane Deformation
The results demonstrate a hinge-opening conformational change between the straight and curved FtsZ protofilament that supports the “hydrolyze-and-bend” model and provides a structural basis for constrictive force generation in bacteria.
Ying Li, Ph.D. Student from Life Sciences Institute, Zhejiang University, is the first author, while Dr. Sheng Ye is the corresponding author of the paper. Yiwen Cheng and Weina Shang from Life Science Institute, Zhejiang University, Dr. Hongwei Wang and Lingyun Zhao (PhD student) from Tsinghua University, and Dr. Kerwyn Casey Huang and Dr. Jen Hsin from Stanford University participate in the work. Shanghai Synchrotron Radiation (SSRF, Beam line BL17U1) provided support for diffraction data collection. The research is funded by the National Natural Science Foundation of China, the Ministry of Science and Technology, the Natural Science Foundation of Zhejiang Province, and the Specialized Research Fund for the Doctoral Program of Higher Education (grant 20110101110122), as well as the Fundamental Research Funds for the Central Universities.



