On February 18,2016, Dr. Haiwei Song’s laboratory published a paper”Structural and Functional Insightsinto the Unwinding Mechanism of Bacteroidessp Pif1” on Cell Reports. Xianglian Zhou ,Wendan Ren and SakshibeeduR.Bharath are the co-first authors, and Dr.Song is the corresponding author of the paper.
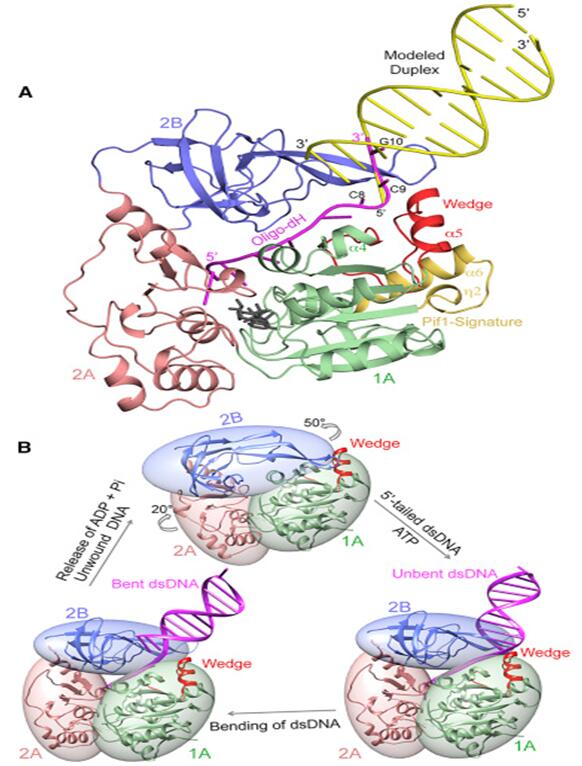
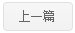
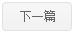
Pif1 is a member of helicases SF1B superfamily,Pif1 can use the energy of ATP hydrolysis to unwinding double strands DNA,DNA/RNA hybrid,G4 DNA.Pif1 is a multi-functional protein and takes part in several biological process in cell.Through the unwinding of the template RNA of telomerase from the telomere DNA,Pif1 can make telomerase free from telomere to maintain the stability of the telomere.In the process of replication and translation ,Pif1 can quickly solve the G4 structure to maintain the stability of genome.Pif1 also has participated in okazaki fragment maturation and DNA damage repair BIR pathway.Here, we reportthe structures of the helicase domain of human Pif1and Bacteroidessp Pif1 (BaPif1) in complex withADP-AlF4–and two different single-stranded DNAs(ssDNAs).Firstly,we found that BaPif1 undergoes a larger conformational change upon DNA binding,especially 2B domain,which is critical for Pif1’s activities.Secondly,the Pif1 signature motif of BaPif1 interacts with the wedge regionin order to stabilize these ssDNAbinding elements, therefore indirectly exerting itsfunctional role. Thirdly,BaPif1 cocrystallized with a taileddsDNA and ADP-AlF4–, resulting in a bound ssDNAbent nearly 90°at the ssDNA/dsDNA junction. Theconformational snapshots of BaPif1 provide insightsinto the mechanism governing the helicase activityof Pif1.