On July 1st 2016, Pinglong Xu laboratory and Xin-Hua Feng laboratory collaborated to publish a research article in Science Advances entitled “PPM1A silences cytosolic RNA sensing and antiviral defense through direct dephosphorylation of MAVS and TBK1.”
Cytosolic RNA/DNA sensing, which is initiated by RIG-I-like receptors or various DNA sensors, elicits primary defense system against viral pathogens. Mitochondria-associated adaptor MAVS and the TBK1 kinase are both essential components for cytosolic RNA sensing and indispensible for interferon production and antiviral defense. Yet, how the status of MAVS and TBK1 activation is controlled by dephosphorylation remains elusive.
Through a functional screen of the human kinome, Xu and Feng laboratories found that metal ion-dependent phosphatase 1A (PPM1A, also known as PP2Cα), depending on its catalytic ability, dampened the RLR-IRF3 axis to silence cytosolic RNA sensing signaling. They demonstrated that PPM1A was an inherent partner of the TBK1/IKKε complex, targeted both MAVS and TBK1/IKKε for dephosphorylation and thus disrupted MAVS-driven formation of signaling complex. In accordance, loss of PPM1A through gene ablation in culture cells or mouse primary macrophages enabled robustly enhanced antiviral responses, and Ppm1a–/– mice resisted to RNA virus attack, whereas transgenic zebrafish expressing PPM1A displayed profoundly increased RNA virus vulnerability. These findings identify PPM1A as the first known phosphatase of MAVS, and elucidate the physiological function of PPM1A in antiviral immunity on whole animals.
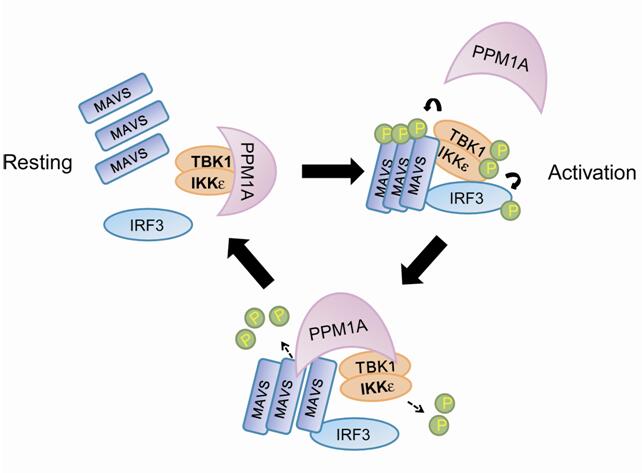 |
| PPM1A-mediated dephosphorylation safegards the machinery for cytosolic antiviral sensing |
This research was accomplished in Life Sciences Institute (LSI) at Zhejiang University, and graduate students Weiwen Xiang and Qian Zhang are co-first authors. Dr. Xin-Hua Feng and Dr. Pinglong Xu are the corresponding authors. This research was supported by the MOST 973 plan (2015CB553800), NSFC Project (81472665), and the National 1000 Young Talents Program. Science Advances is the offspring of Science and the first open access online journal of AAAS, which publishs significant, innovative original research that advances the frontiers of science and extends the standards of excellence established by Science.
Text link:http://advances.sciencemag.org/content/2/7/e1501889



