On October 4, 2016, a research article entitled “SUMO Modification Reverses Inhibitory Effects of Smad Nuclear Interacting Protein-1 in TGF-beta Responses” was published by the Xin-Hua Feng Lab in the online version of Journal of Biological Chemistry.
Smad nuclear interacting protein-1 (SNIP1) is one of the transcription repressors that inhibit TGF-beta signaling through disrupting the transcriptional activator complex of Smads, the central mediator of TGF-beta signaling, and its interaction with transcription co-activator p300. However, how SNIP1 activity is regulated remains enigmatic.
Protein post-translational modification is one of the essential mechanisms to regulate protein stability, activity and subcellular localization. Our detailed biochemical studies have revealed that SNIP1 is a SUMOylated protein, which is consistent with the presence of several potential SUMOylation consensus motifs on SNIP1. There are two highlight points in the current study.
First, biochemical and mutagenesis studies revealed that SUMOylation of SNIP1 is a phosphorylation-dependent SUMOylation chain reaction. The authors found that SUMOylation of SNIP1 occurs on three lysine residues, i.e. Lys-5, Lys-30 and Lys-108 and phosphorylation at Ser-35 controls the initiation of SUMOylation of SNIP1. Interestingly, SUMOylation of SNIP1 occurs in a sequential order. Upon Ser-35 phosphorylation, Lys-30 becomes SUMOylated, which controls the subsequent SUMOylation at Lys-108. SUMOylation at Lys-108 allows the final SUMOylation at Lys-5. Therefore, the authors discovered for the first time that SUMOylation occurs in a stepwise manner.

SUMOylation of SNIP1 is a phosphorylation-dependent SUMOylation chain reaction.
Secondly, functional assays showed that SNIP1 inhibits TGF-beta-induced transcription, cell migration and invasion, and significantly SUMO modification reverses the inhibitory effects of SNIP1 on the TGF-beta responses. Mechanically, SUMO modification impairs the activity of SNIP1 in disrupting formation of the Smad complex and attenuating the recruitment of p300 to Smad proteins. Consequently, SUMO modification disables SNIP1 in suppressing TGF-beta signaling.
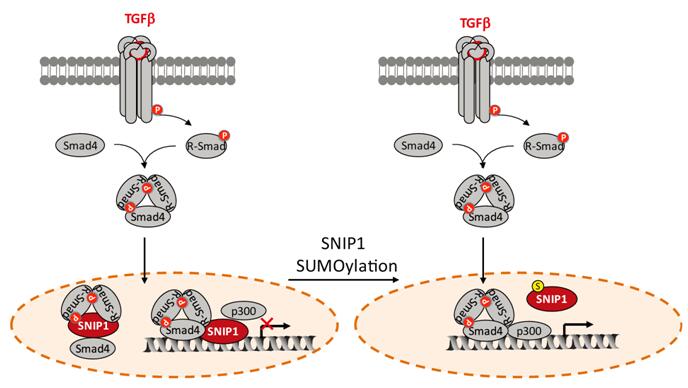
SUMO modification regulates the function of SNIP1 in TGF-beta signaling pathway
The project was supported by the Key Program of the Ministry of Sciences and Technology (2012CB966600), National Natural Science Foundation of China Grants (91540205, 31571447), Major Program of the National Natural Science Foundation of China (31090360), the Fundamental Research Funds for the Central Universities, US DoD grant (DAMDW81XWH-15-1-0650) and NIH grants (R21CA209007, R01GM63773, R01AR053591, R01CA108454, and R01DK073932).
Link:http://www.jbc.org/content/early/2016/10/04/jbc.M116.755850.long