On January 13, 2017, a research article titled “Activation of c-Abl kinase potentiates the anti-myeloma drug lenalidomide by promoting DDA1 recruitment to the CRL4 ubiquitin ligase” from Cang Lab was published online in Journal of Biological Chemistry.
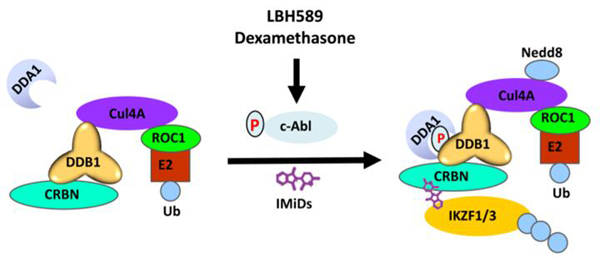
Lenalidomide is an immunomodulatory drug (IMiD) commonly used in combination with other classes of drugs to treat multiple myeloma (MM), but the molecular rationale underlying these combination therapies is largely unknown. Cullin Ring Ligase 4 (CRL4), a complex of Cul4 and DDB1, regulates cell cycle, DNA damage repair, and chromatin replication by targeting a variety of substrates for ubiquitination. Lenalidomide hijacks the CRL4CRBN ubiquitin ligase to degrade essential lymphoid transcription factors IKZF1 and IKZF3 in MM cells.
c-Abl was reported to bind and phosphorylate DDB1, an adaptor protein of the CRL4 complex, but the exact role of c-Abl in regulating CRL4 is not clear. Here we report that the c-Abl non-receptor kinase phosphorylates DDB1 at residue Y316 to recruit a small regulatory protein DDA1, leading to increased substrate ubiquitination. Pharmacological inhibition or genetic ablation of the Abl-DDB1-DDA1 axis decreases the ubiquitination of CRL4 substrates, including IKZF1 and IKZF3 in lenalidomide-treated multiple myeloma cells. Importantly, panobinostat, a recently approved anti-myeloma drug, and dexamethasone enhance lenalidomide-induced substrate degradation and cytotoxicity by activating c-Abl, therefore providing mechanism underlying their combination with lenalidomide to treat multiple myeloma. There are two highlights in this study.
First, biochemical and mutagenesis studies revealed that c-Abl phosphorylates multiple tyrosine residues of the DDB1 protein (Tyr-316, Tyr-182, Tyr-718) but only phosphorylation at Tyr-316 promotes the recruitment of DDA1. Activation of c-Abl kinase promotes the degradation of CRL4 substrates, including histone H3, IKZF1, IKZF3 and CK1α.
Second, genetic and cellular assays showed that two anti-myeloma drugs, panobinostat and dexamethasone, enhance lenalidomide-induced death of MM cells by activating the c-Abl non-receptor tyrosine kinase.
This work was supported in part by funds from the National 973 Plan for Basic Research (2015CB553803), National Natural Science Foundation (91429302), and Fundamental Research Funds for the Central Universities and Key Construction Program of the National ‘985’ Project. Shaobing Gao is a graduate student in LSI and first author of the paper.



