On March 23, 2017, Fangwei Wang Lab published a research Article in Current Biology entitled "The N-Terminal Non-Kinase Domain-Mediated Binding of Haspin to Pds5B Protects Centromeric Cohesion in Mitosis".
Sister-chromatid cohesion, mediated by the multi-subunit cohesin complex, must be precisely regulated to prevent chromosome mis-segregation. In prophase and prometaphase, while the bulk of cohesin on chromosome arms is removed by its antagonist Wapl, cohesin at centromeres is retained to ensure chromosome biorientation until anaphase onset. It remains incompletely understood how centromeric cohesin is protected against Wapl in mitosis.
In this study, the authors show that the mitotic histone kinase Haspin binds to the cohesin regulatory subunit Pds5B through a conserved YGA/R motif in its non-catalytic N-terminus, which is similar to the recently reported YSR motif-dependent binding of Wapl to Pds5B. Knockout of Haspin or disrupting Haspin-Pds5B interaction causes weakened centromeric cohesion and premature chromatid separation, which can be reverted by centromeric targeting of a N-terminal short fragment of Haspin containing the Pds5B-binding motif, or by preventing Wapl-dependent cohesin removal. Conversely, excessive Haspin capable of binding Pds5B displaces Wapl from Pds5B and suppresses Wapl activity, and largely bypasses the Wapl antagonist Sgo1 for cohesion protection.
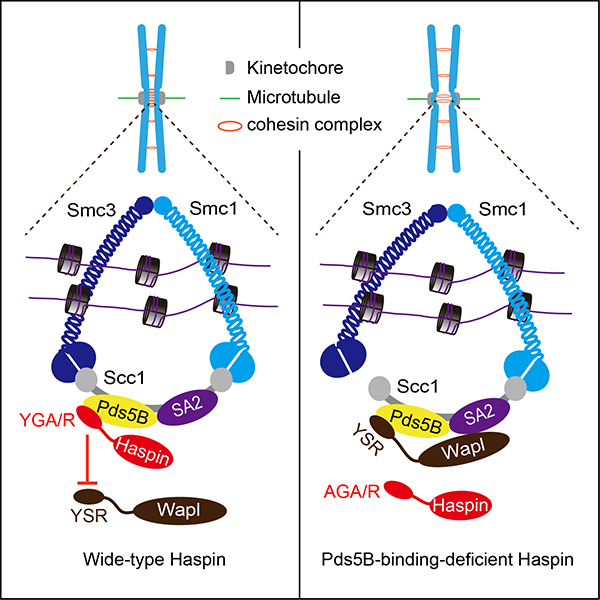
Taken together, these data indicate that the Haspin-Pds5B interaction is required to ensure proper sister-chromatid cohesion, likely through antagonizing Wapl-mediated cohesin release from mitotic centromeres. This study helps rationalize the apparent complexity of the centromere signaling network, and will have broader implications for the understanding of chromosome dynamics during mitosis.
Graduate students Linli Zhou and Cai Liang are co-first authors of this study, and Professor Fangwei Wang is the corresponding author. This work was supported by grants from the National Natural Science Foundation of China, a National Thousand Young Talents Award, and the Natural Science Foundation of Zhejiang Province.
Text link:http://www.cell.com/current-biology/fulltext/S0960-9822



