On June 16th 2017, Long Zhang laboratory published a research article in Cell Death & Differentiation entitled “SUMO-triggered ubiquitination of NR4A1 controls macrophage cell death”, which shows that nuclear receptor NR4A1 is sumoylated by SUMO2/3.
Nuclear receptor NR4A1 has been implicated as a key regulator in a wide range of pathophysiological responses. As an immediate early response gene, NR4A1 can be rapidly and potently induced by a variety of stimuli. Its induction is followed by its rapid degradation, but the mechanism by which NR4A1 is degraded remains poorly understood.
Here we show that nuclear receptor NR4A1 is sumoylated by SUMO2/3. Upon poly-SUMO modification, NR4A1 can be targeted by the SUMO-dependent E3 ubiquitin ligase RNF4 for polyubiquitination and subsequent degradation. The SUMO E3 ligase PIAS3 promotes SUMOylation and polyubiquitination of NR4A1, while the SUMO protease SENP1 acts to de-conjugate SUMO. We demonstrate that this pathway is important for rapid degradation of NR4A1 after induced by stress. Moreover, we identify two SUMO modification sites in NR4A1 that are critical for maintaining low levels of NR4A1 expression. Mutation of these two NR4A1 SUMO modification sites enhances the stability of NR4A1. Importantly, we show that SUMOylation is critical in controlling NR4A1 function in inflammatory cytokine signaling and controlling macrophage cell death. SUMOylation and subsequent ubiquitination on NR4A1 mitigates its inhibition of innate immune signaling, such as TNF-α- and IL-1β-induced NF-κB activation.
This mechanism of sequential SUMOylation and ubiquitination, which together control the degradation of NR4A1, could be exploited for the therapeutic treatment of diseases with NR4A1 involvement.
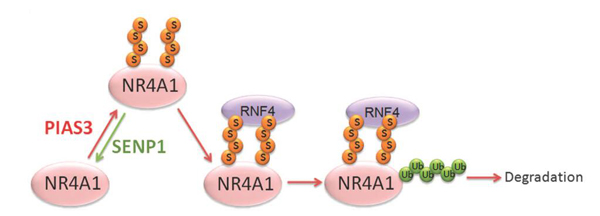
Figure: SUMO modification-mediated NR4A1 ubiquitination and degradation
The first authors of this article are Prof. Long Zhang and Dr. Feng Xie. The corresponding authors are Prof. Long Zhang and Prof. Fangfang Zhou.
Link: https://www.nature.com/cdd/journal/vaop/ncurrent/full/cdd201729a.html



