On November 14, 2017, the Fangwei Wang Lab at Life Sciences Institute, Zhejiang University, published a research paper in EMBO Reports entitled "A kinase-dependent role for Haspin in antagonizing Wapl and protecting mitotic centromere cohesion".
Sister-chromatid cohesion mediated by the cohesin complex is fundamental for precise chromosome segregation in mitosis. Through binding the cohesin subunit Pds5, Wapl releases the bulk of cohesin from chromosome arms in prophase, whereas centromeric cohesin is protected from Wapl until anaphase onset. Strong centromere cohesion requires centromeric localization of the mitotic histone kinase Haspin, which is dependent on the interaction of its non-catalytic N-terminus with Pds5B. It remains unclear how Haspin fully blocks the Wapl-Pds5B interaction at centromeres.
In this study, Professor Fangwei Wang and his students show that the C-terminal kinase domain of Haspin (Haspin-KD) binds and phosphorylates the YSR motif of Wapl (Wapl-YSR), thereby directly inhibiting the YSR motif-dependent interaction of Wapl with Pds5B. Cells expressing a Wapl-binding-deficient mutant of Haspin or treated with Haspin inhibitors show centromeric cohesion defects. Phospho-mimetic mutation in Wapl-YSR prevents Wapl from binding Pds5B and releasing cohesin. Forced targeting Haspin-KD to centromeres partly bypasses the need for Haspin-Pds5B interaction in cohesion protection. Taken together, these results indicate a kinase-dependent role for Haspin in antagonizing Wapl and protecting centromeric cohesion in mitosis.
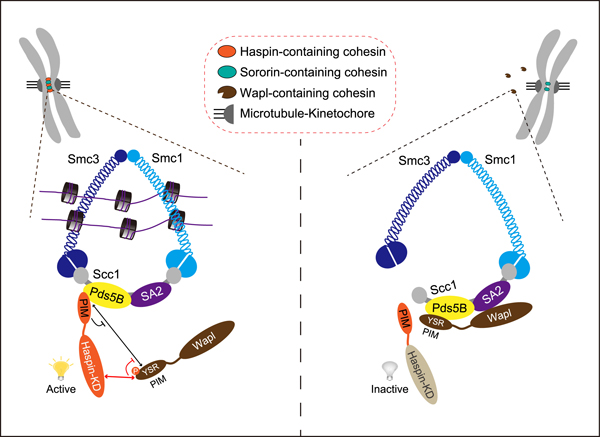
Schematic depiction of the role for Haspin in protecting centromeric cohesin during mitosis.
We propose that there are different pools of centromeric cohesin complexes in which Pds5B binds to either Haspin or Sororin, another Wapl antagonist. The interaction of Pds5B with the N-terminal PIM of Haspin enables the centromeric localization of Haspin, which can competitively interfere with the YSR motif-dependent binding of Wapl to Pds5B. The centromeric Haspin uses its C-terminal kinase domain to bind and phosphorylate Wapl at the YSR motif, thereby directly inhibiting the Wapl-Pds5B interaction at mitotic centromeres and preventing Wapl-mediated release of the Haspin-containing cohesin complex.
Graduate students Cai Liang and Qinfu Chen are co-first authors of this study, and Professor Fangwei Wang is the corresponding author. This work was supported by grants from National Key Research and Development Program of China, National Natural Science Foundation of China, and the National Thousand Young Talents Award.
Link:http://embor.embopress.org/content/early/2017/11/14/embr.201744737



