Yongqun Zhu laboratory, PNAS: Structural insights into the roles of the IcmS-IcmW complex in the type IVb secretion system of Legionella pneumophila
On December 4, 2017, Dr. Yongqun Zhu laboratory online published a research paper in PNAS entitled “Structural insights into the roles of the IcmS-IcmW complex in the type IVb secretion system of Legionella pneumophila”, which provides molecular insights into the dual roles of the IcmS-IcmW complex in T4BSSs.
The type IVb secretion system (T4BSS) of Legionella pneumophila is amultiple-component apparatus that delivers ~300 virulent effectorproteins into host cells. The injected effectors modulate host cellularprocesses to promote bacterial infection and proliferation. IcmS andIcmW are two conserved small, acidic adaptor proteins that form abinary complex to interact with many effectors and facilitate theirtranslocation. IcmS and IcmW can also interact with DotL, an ATPaseof the type IV coupling protein complex (T4CP). However, how IcmS�IcmW recognizes effectors, and what the roles of IcmS-IcmW are inT4BSSs are unclear. In this study, we found that IcmS and IcmW forma 1:1 heterodimeric complex to bind effector substrates. Both IcmSand IcmW adopt new structural folds and have no structural similaritieswith known effector chaperones. IcmS has a compact globalstructure with an α/β fold, while IcmW adopts a fully α-folded, relativelyloose architecture. IcmS stabilizes IcmW by binding to its twoC-terminal α-helices. Photocrosslinking assays revealed that theIcmS-IcmW complex binds its cognate effectors via an extended hydrophobicsurface, which can also interact with the C terminus ofDotL. A crystal structure of the DotL-IcmS-IcmW complex revealsextensive and highly stable interactions between DotL and IcmS-IcmW. Moreover, IcmS-IcmW recruits LvgA to DotL and assemblesa unique T4CP. These data suggest that IcmS-IcmW also functions asan inseparable integral component of the DotL-T4CP complex in thebacterial inner membrane. This study provides molecular insights intothe dual roles of the IcmS-IcmW complex in T4BSSs.
Jianpo Xu and Dandan Xu are the first authors, Dr. Yongqun Zhu and Dr. Yan Zhou are the corresponding authors of this paper. We thank Drs. Peng Chen and Zengyi Chang for providing plasmids and reagents for photocrosslinking assays and Dr. Lijie Wuandthe staffs at beamlines BL17U1 and BL18U1 of the Shanghai Synchrotron Radiation Facility and the National Center for Protein Science Shanghai forassistance with diffraction data collection. This research was supported by National Natural Science Foundation of China Grantsand the Fundamental Research Funds for the Central Universities.Dr. Yongqun Zhu was awarded the Newton Advanced Fellowship by the Royal Society.
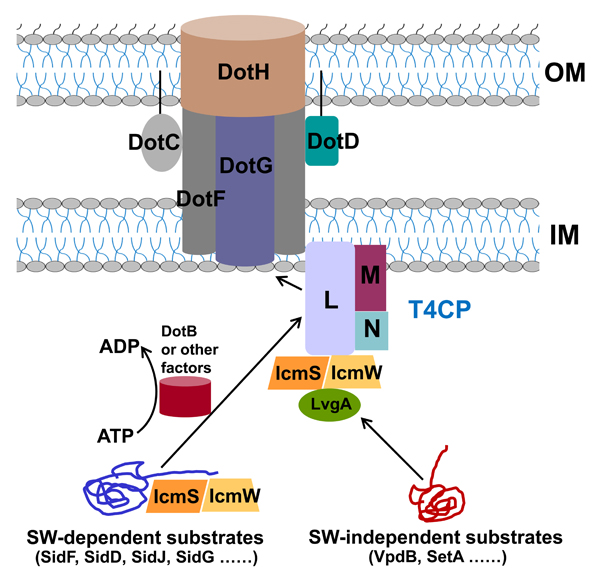
Figure. The molecular mechanism and the dual roles of IcmS-IcmW in the type IVb secretion system of Legionella pneumophila



