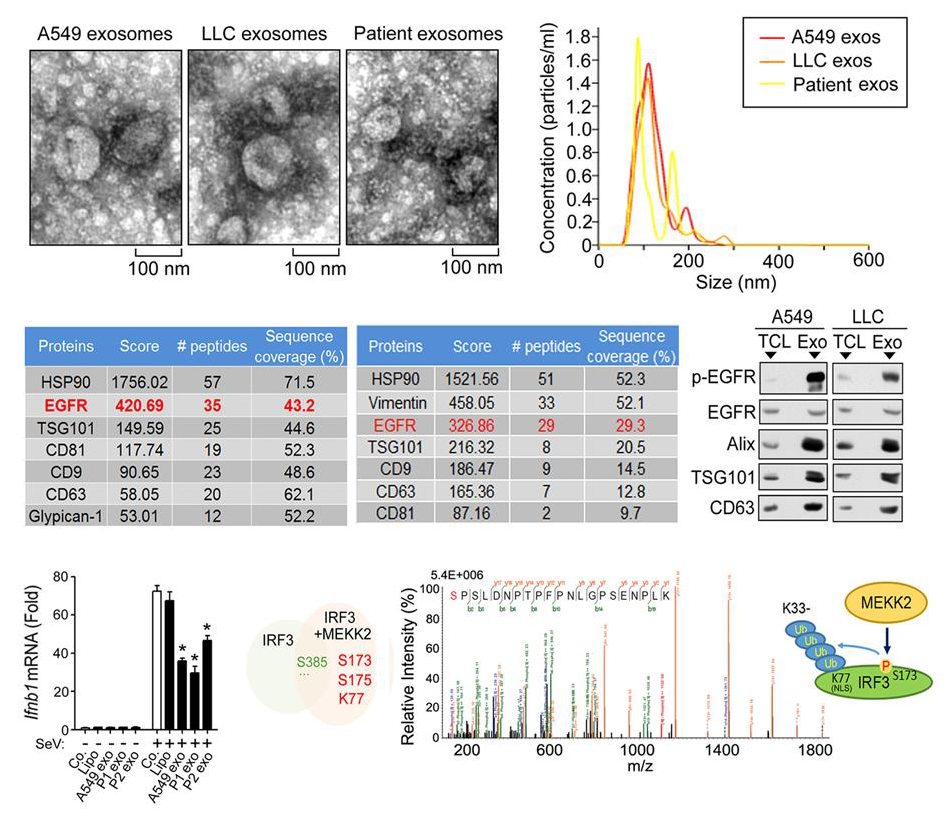
Immunological defects can be caused by various conditions and diseases, including, for example, malignancy, organ or stem cell transplantation, systemic vasculitis, and connective tissue disease. In patients with lung cancer that had not undergone any procedures such as intramuscular injection or chemotherapy, we observed impaired induction of IFN-β in relative to its induction in patients who did not have cancer. We showed that tumor cells were able to repress host innate immunity by secreting and transferring EGFR+exosomes to innate immune cells. Active EGFR stimulated the host’s MEKK2, which then phosphorylated IRF3 at Ser173; this led to repression of the activation of IRF3 and type I interferon and weakening of the host’s pathogen-defense ability.
Activated EGFR recruits various cytoplasmic proteins that transduce and/or regulate its function. The EGFR pathway induces the proliferation, differentiation, migration, adhesion and survival of cells through various interacting signaling pathways, including RAS�RAF�MAPK, PI3K and JAK�STAT. Cancer cells can transfer receptor tyrosine kinases such as phosphorylated EGFR and human EGFR2 (HER-2) to monocytes via exosomes, which prolongs monocyte survival and changes the microenvironment of cancers. Here we demonstrated that multiple types of cancer cells secreted EGFR+ exosomes. In addition, our results showed that cancer cells were able to deliver activated EGFR to host immune cells, which interfered with the innate immunity via MEKK2-mediated deregulation of IRF3. MEKK2 is a serine-threonine kinase that belongs to the MEKK-STE11 subgroup of the MAP3K family of kinases. After upstream activation, MEKK2 can activate the ERK5 and JNK kinase pathways. Here we showed that MEKK2 mediated phosphorylation of IRF3 at Ser173 independently of ERK5 and JNK in cells in vivo and phosphorylated IRF3 at Ser173 directly in vitro. Furthermore, we found that, via phosphorylation of IRF3 at Ser173, MEKK2 inhibited the virus-induced translocation of IRF3 to the nucleus by triggering K33-linked poly-ubiquitination on the IRF3 NLS motif. Homozygous replacement of endogenous wild-type IRF3 with the mutant IRF3 S173A resulted in very low levels of K33-linked poly-ubiquitination of IRF3 after activation of MEKK2 or treatment with exosomes and increased the translocation of IRF3 to the nucleus and expression of IFNB1 after viral stimulation.
Graduate students Liang Gao, Lin Wang, Tong Dai, Zhengkui Zhang and Postdoc Ke Jin are the co-first authors of this study. Prof. Long Zhang and prof. Fangfang Zhou are the corresponding authors.This work was supported by a special program from Ministry of Science and Technology of China(2016YFA0502500), the Chinese National Natural Science Funds (31701232,31571460,31471315,31671457和91753139), PCSIRT, Jiangsu National Science Foundation (BK20150354 and Zhejiang outstanding youth fund (LR14C070001).
Link:https://www.nature.com/articles/s41590-017-0043-5



