On July 17, 2018, the Fangwei Wang Lab at Life Sciences Institute, Zhejiang University, published a research paper in Cell Reports entitled " WAC Promotes Polo-Like Kinase 1 Activation for Timely Mitotic Entry".
Polo-like kinase 1 (Plk1) plays a key role in many steps of mitotic cell division ranging from mitotic entry to cytokinesis. Plk1 consists of an N-terminal kinase domain and a C-terminal polo-box domain (PBD). The PBD usually binds to a phosphorylated protein motif which can target Plk1 to its various subcellular localizations, and also functions as a negative regulator of the kinase domain in Plk1. Plk1 can be activated through phosphorylation of its activation loop in the kinase domain by upstream kinases, possibly through disrupting its auto-inhibitory interaction with PBD.
Plk1 activity starts to increase during G2 phase and peaks in mitosis. During G2 phase of human cells, Aurora-A kinase (AurkA) cooperates with its co-factor Bora to phosphorylate Plk1 at Thr-210 in its activation loop, which is important for Plk1 activation and the timely entry into mitosis. Several lines of evidence suggest that Plk1 phosphorylation at T210 (Plk1-pT210) occurs at the centrosome, which is consistent with the cytoplasmic localization of Bora in interphase cells. However, Plk1 activity is first seen in the nucleus of early G2 cells. The mechanism underlying Plk1 activation during the G2 to mitosis (G2/M in short) transition remains incompletely understood.
In this study, Feifei Qi et al. report that the activation of Plk1 requires WAC, a WW domain-containing adaptor protein with a coiled-coil region which predominantly localizes to the nucleus in interphase. Cyclin-dependent kinase 1 (Cdk1) phosphorylates WAC, priming its direct interaction with the polo-box domain of Plk1. Knockdown of WAC compromises Plk1 activity and delays mitotic entry. These defects are rescued by exogenous expression of wild-type WAC but not the Plk1-binding-deficient mutant. WAC also binds AurkA and can enhance Plk1 phosphorylation by AurkA in vitro. Taken together, these results indicate an important role for WAC in promoting Plk1 activation and the timely entry into mitosis.
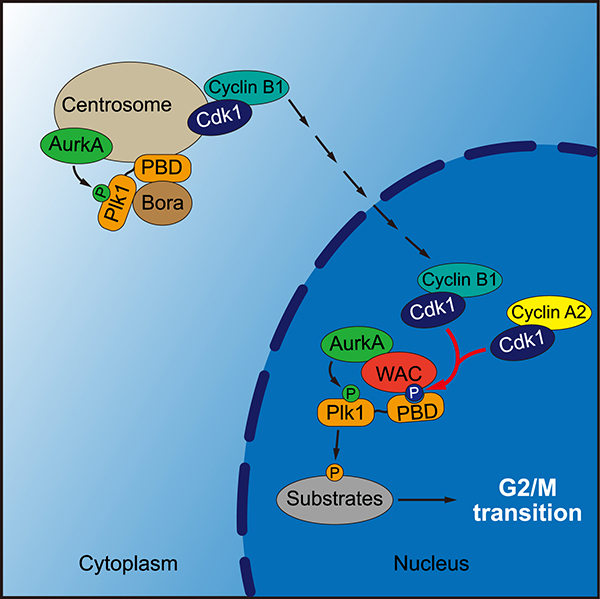
Model for Plk1 activation by WAC and AurkA in the nucleus of G2 phase cells
Graduate student Feifei Qi is the first author of this study, and Professor Fangwei Wang is the corresponding author. Professor Jun Huang is the collaborator. This work was supported by grants from National Key Research and Development Program of China, the National Natural Science Foundation of China, the Natural Science Foundation of Zhejiang Province, and the National Thousand Young Talents Award.
Link:https://www.cell.com/cell-reports/fulltext/S2211-1247(18)31029-5



