A study entitled “Cryo-EM structure of the human α5β3 GABAA receptor” was published online in the Cell Research on August 23, 2018. In collaboration with the Center of Cryo Electron Microscopy at Zhejiang University, Professor Sheng Ye’s team from the Life Sciences Institute have used single particle cryo-EM coupled with nanobody to determine the structure of a heteropentameric α5β3 GABAA receptor in an open state, at an average resolution of 3.51 Å. The structure reveals a subunit stoichiometry of one α5 and four β3 subunits. This structure is the basis for subsequent large-scale biophysical and pharmacological studies of GABAA receptors.
γ-aminobutyric acid (GABA) is a major inhibitory neurotransmitter in central nervous system (CNS). γ-aminobutyric acid type A (GABAA) receptors, located in synaptic and extrasynaptic, mediate rapid inhibitory neurotransmission by opening a chloride selective pore in response to binding of GABA, and thus are vital for controlling excitability in the brain. Dysfunctional GABAA receptors are directly involved in the pathogenesis of many neurologic diseases and psychiatric disorders, such as epilepsy and schizophrenia. GABAA receptors are modulated, directly activated or inhibited by over hundreds of pharmacologically and clinically important compounds of different structural classes, including GABA, benzodiazepines, neuroactive steroids, propofol, ethanol, picrotoxin, bicuculline. As a member of Cys loop-type pentameric ligand-gated ion channel (pLGICs) superfamily, GABAA receptors are heteropentamers assembled from a repertoire of 19 different subunits (a, β, g, d, e, q, p, and r subunits), giving rise to a spectrum of GABAA receptor subtypes with different subunit compositions and arrangements, as well as distinct biophysical and pharmacological properties. Although the subunit stoichiometries and arrangements of GABAA receptor subtypes have been intensively investigated in the last two decades, the assembling principles of these receptor subtypes remain unknown. Moreover, GABA ligand binding site in the α/β subunit interface and the working mechanism of the receptors are unclear.
To elucidate the assembly principle and the ligand-gating mechanism of GABAA receptors containing both α and β subunits, we determined the cryo-EM structure of human α5β3 GABAA receptor, revealing a subunit stoichiometry of one α5 and four β3 subunits.
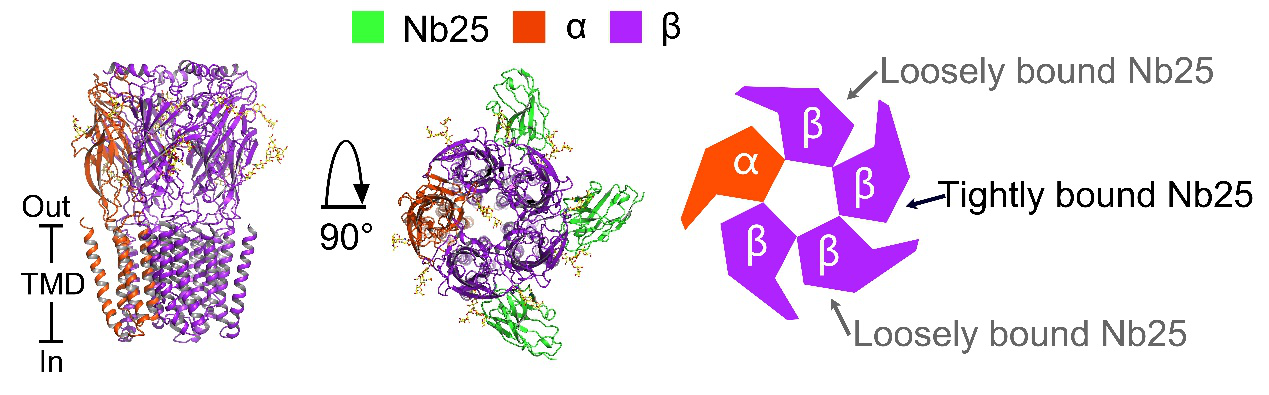
Figure 1: Side view, top view, schematic top-down view of the cryo-EM structure of the human a5b3 GABAA receptor
The receptor represents an activated conformation around an open pore, with a GABA in the ligands binding site. These structural features initially revealed the ligand gating mechanism of the GABAA receptor.
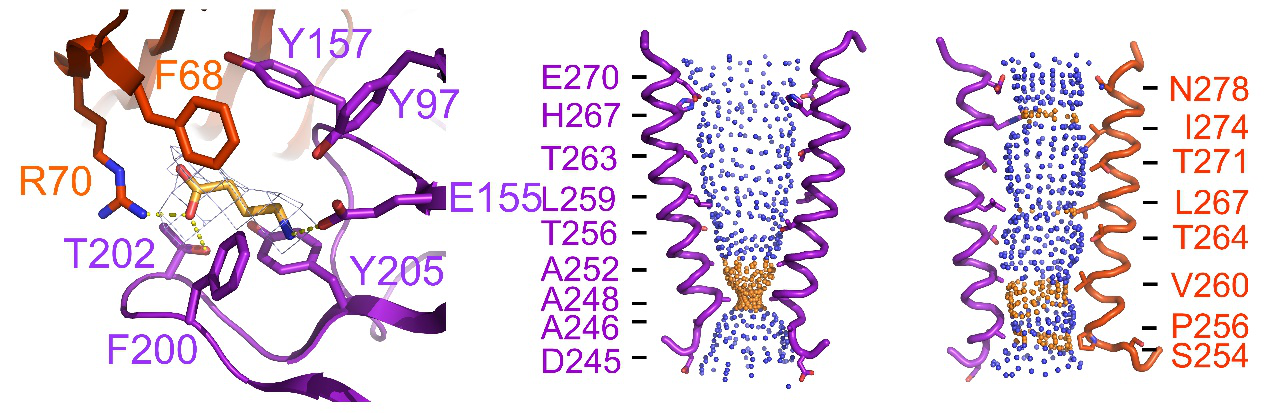
Figure 2: GABA binding site; shape of the ion permeation pathway: homopentameric b3 GABAA receptor in the desensitized state (left) and that of heteropentameric α5β3 GABAA receptor in the open state(right)
The project was completed by Sheng Ye’s Laboratory at Zhejiang University, Center of Cryo Electron Microscopy of Zhejiang University, Z. Hong Zhou’s Laboratory of University of California, Los Angeles and Guo-Qiang Bi’s Laboratory of China University of Science and Technology. Si Liu, Lingyi Xu, Fenghui Guan, PhD students from Ye’s lab, are co-first authors. Prof. Sheng Ye, Associate Prof. Xiaokang Zhang, Prof. Z. Hong Zhou are the corresponding authors. This work was founded by Ministry of Science and Technology, the National Natural Science Foundation of China, Fundamental Research Funds for the Central Universities and National Outstanding Young Scholar Science Foundation.



