On August 29th, 2018, Professor JinJin‘s group published a research paper entitled “CRL4DCAF2 is required for mature T-cell expansion via Aurora B-regulated proteasome activity” in Journal of Autoimmunity. This article reveal CRL4DCAF2 as a critical mediator controlling M phase exit in activated T cells and autoimmune responses.
The clonal expansion of antigen-specific T cells is essential for the induction of effective adaptive immune responses. The cycling of T cells is tightly controlled by the ordered expression of cyclin/CDK complexes. Recently, a novel E3 ubiquitin ligase CRL4DCAF2 has been characterized as a master regulator that prevents rereplication of DNA in S phase. CRL4DCAF2 promotes the ubiquitin-dependent proteolysis of various substrates such as CDT1, p21, and Set8.
We useCRL4DCAF2 T cell-specific knockout miceto clarify the underlying mechanism of DCAF2 in vivo, especially in the immune system. The specific knockout mice display impaired peripheral T cell maintenance and reduced severity of various autoimmune diseases.IL-2 has long been recognized as a major T cell growth factor and a regulator of G1/S phase progression. RNA-seq analyses indicated that IL-2 induction was severely abolished in DCAF2-deficient T cells. Moreover, exogenous IL-2 could not restore the proliferative capacity of DCAF2-deficient naïve T cells. Our study also demonstrated that neither p21 nor c-Myc is involved in CRL4DCAF2-mediated T cell expansion and cell cycle progression.
Aurora B has been identified as a pivotal kinase for enhancing 26S proteasome activity, which is indispensable for the CRL4DCAF2-dependent proliferation of T cells. In this study, the Aurora B-phosphorylated 26S proteasome was shown to play an important role in cell cycle regulation. The 26S proteasome contains more than 300 phosphorylation sites that are dynamically regulated during the cell cycle.Here, we show that the 26S proteasome is also dynamically phosphorylated by Aurora B in a cell cycle-regulated manner. Silencing of Aurora B in T cells severely impaired 26S proteasome activity and caused M phase arrest. Our findings demonstrate a novel signaling network that regulates primary T cell proliferation and has profound therapeutic implications for T cell-mediated autoimmune diseases.
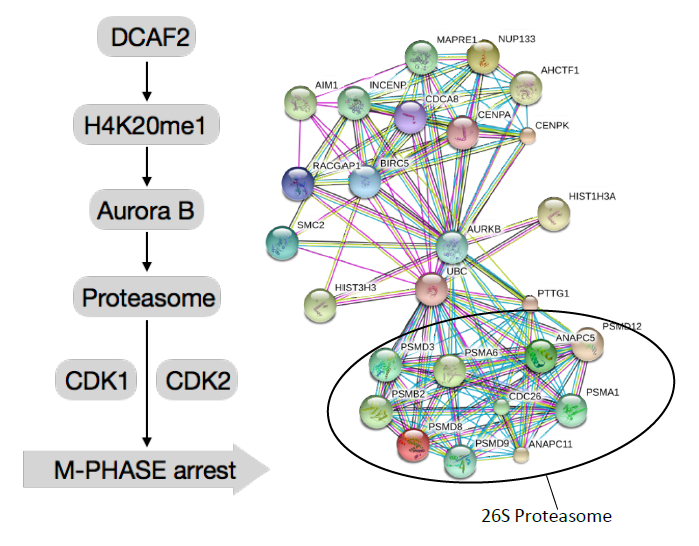
Figure1,DCAF2 restricts H4K20me1 modifications at Aurk promoter loci in activated T cells during the M phase, which regulates 26S proteasome activity, suggested that Dcaf2 deficiency causes M phase arrest through proteasome-dependent mechanisms in peripheral T cells.
Ph.D. students Keqi Fan and Wang Fei in Dr. Jin’s group are co-first authors. Professor JinJin and Professor Xing Gio are corresponding authors of this article.
Links:https://www.sciencedirect.com/science/article/pii/S0896841118303676



