On November 21, 2018, Professor Long Zhang’s group published a research paper entitled "OTUB2 promotes cancer metastasis via Hippo independent activation of YAP and TAZ". This article revealed how the malignant tumor manages to maintain YAP/TAZ protein stability when the Hippo pathway is still intact.
The transcriptional regulators YAP and TAZ play important roles in development, physiology and tumorigenesis and are negatively controlled by the Hippo pathway. Upon activation of Hippo signaling, YAP and TAZ are phosphorylated by LATS1/2 and Casein 1δ/ε kinases, leading to poly-ubiquitination by SCF E3 ubiquitin ligase and proteasomal degradation. Although YAP/ TAZ proteins are frequently activated in human malignancies in which the Hippo pathway is still active, it is yet unknown whether YAP/TAZ can be stabilized via deubiquitination and thereby escape from the negative Hippo control. Here, by a gain-of-function cancer metastasis screen we discovered OTUB2 as a cancer stemness and metastasis-promoting factor that deubiquitinates and activates YAP/TAZ. We found OTUB2 to be poly-SUMOylated on Lysine 233 and this SUMOylation enables it to bind YAP/TAZ. We also identified an as yet unknown SUMO-interacting motif (SIM) in YAP and TAZ required for their association with SUMOylated OTUB2. Importantly, EGF and oncogenic KRAS induce OTUB2 poly-SUMOylation and thereby activating YAP/TAZ. Our results not only establish OTUB2 as an essential modulator of YAP/TAZ, but also reveal a novel regulatory mechanism via which YAP/TAZ activity is induced by oncogenic KRAS.
Highlights :
1. OTUB2 enhances metastasis through Hippo independent activation of YAP/TAZ signaling.
2. Poly-SUMOylation is required for OTUB2 to interact with and deubiquitinate YAP/TAZ.
3. YAP/TAZ contain a SUMO-interacting motif (SIM) required for binding SUMOylated OTUB2.
4. EGF treatment or KRAS mutation stabilizes YAP/TAZ by stimulating OTUB2 SUMOylation.
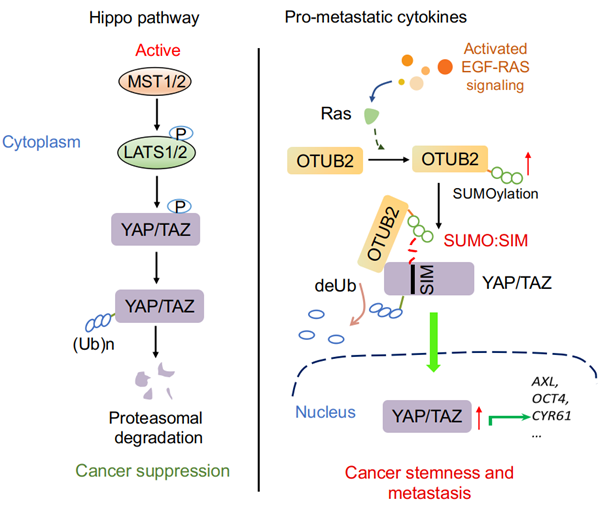
Working model of SUMOylated OTUB2-mediated YAP/TAZ deubiquitination: this is the first report of DUB-mediated control of YAP/TAZ proteins, and also the first report that YAP/TAZ are regulated by KRAS via a mechanism that was so far completely unknown. They answered how YAP and TAZ proteins are maintained and activated in human malignancies.
The project was completed by Long Zhang’s Lab at Zhejiang University. The Ph.D. student Zhengkui Zhang is the first author. Prof. Long Zhang is the corresponding author.
This work was supported by a special program from Ministry of Science and Technology of China (2016YFA0502500 to L.Z.), the Chinese National Natural Science Funds (31471315, 31671457, 31741086 and 91753139 to L.Z.), Zhejiang outstanding youth fund (LR14C070001 to L.Z.), the major social development projects of Zhejiang S&T Major Projects (No. 2015C03045 to M.Z.) and grants from the National Institutes of Health (CA196878, DE15964, and GM51586 to K.-L.G.).
Links:https://www.cell.com/molecular-cell/fulltext/S1097-2765(18)30890-6



