On December6,2018, the Fangwei Wang Labat Life Sciences Institute, Zhejiang University, published a research paper in Journal of Biological Chemistry entitled "Aurora B kinase activity-dependent and -independent functions of the chromosomal passenger complex in regulating sister chromatid cohesion".
Chromosomal instability resulting from chromosome missegregation during mitosis is a hallmark of cancer cells and contributes to tumorigenesis. The fidelity of chromosome segregation is guaranteed by a sophisticated mechanism that ensures timely resolution of sister chromatid cohesion, correct attachment of kinetochores to microtubules, and proper response of spindle assembly checkpoint monitoring the status of kinetochore-microtubule attachments which allows chromosome segregation only when all chromosomes have completed bi-orientation on the metaphase plate. The chromosomal passenger complex (CPC) is a master regulator of mitosis. The CPC consists of the kinase subunit Aurora B, the scaffold subunit INCENP and two regulatory subunits Survivin and Borealin. These proteins play specific roles in controlling the localization and function of the CPC. During early mitosis, Survivin and Borealin associate with the N-terminal CEN domain of INCENP and mediate the centromeric localization of CPC by directly binding to phosphorylated histone tails. The C-terminus of INCENP binds to Aurora B through an IN-box domain, leading to an allosteric activation of Aurora B. Heterochromatin protein 1, which binds to INCENP and distributes along with the CPC to various locations throughout mitosis, can be considered the fifth subunit of the CPC. However, the functional significance of HP1 in the CPC remains incompletely understood.
In this study, Qi Yi and Qinfu Chen et al. show that Aurora B kinase activity contributes to centromeric cohesion protection partly through promoting kinetochore localization of the kinase Bub1. Interestingly, disrupting the interaction of INCENP with HP1 in HeLa cells selectively weakens cohesion at mitotic centromeres without detectably reducing the kinase activity of Aurora B. Thus, through this INCENP-HP1 interaction, the CPC also protects centromeric cohesion independently of Aurora B kinase activity. Moreover, the requirement for the INCENP-HP1 interaction in centromeric cohesion protection can be bypassed by tethering HP1 to centromeres or by depleting the cohesin release factor Wapl. The authors provide further evidence suggesting that the INCENP-HP1 interaction protects centromeric cohesion by promoting the centromere localization of Haspin, a protein kinase that antagonizes Wapl activity at centromeres. Taken together, this study identifies Aurora B kinase activity-dependent and -independent roles for the CPC in regulating centromeric cohesion during mitosis in human cells.
Graduate student Qi Yiand Qinfu Chen areco-first authors of this study. Professor Fangwei Wang is the corresponding author.This work was supported by grants from National Key Research and Development Program of China, National Natural Science Foundation of China,Natural Science Foundation of Zhejiang Province, Newton Advanced Fellowship, and Fundamental Research Funds for the Central Universities.
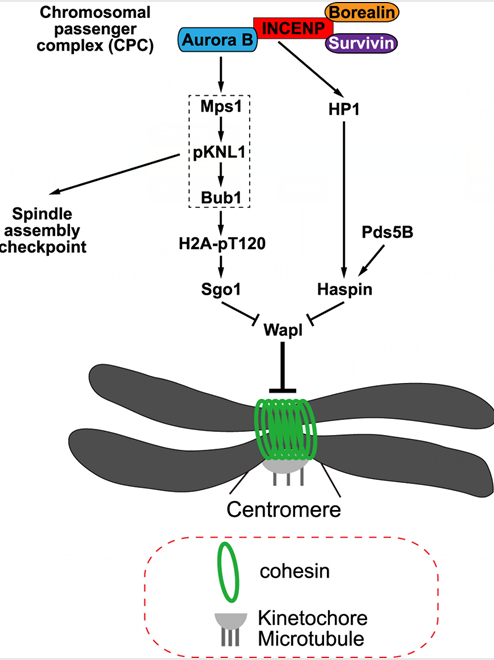
Figure: Model for the Aurora B kinase activity-dependent and -independent roles for the CPC in protecting sister chromatid cohesion at mitotic centromeres.
Links:http://www.jbc.org/content/early/2018/12/06/jbc.RA118.005978.abstract



