On January 21, 2019, Nature Cell Biology published a research article entitled “ALK phosphorylates SMAD4 on tyrosine to disable TGF-β tumor suppressor functions” by Dr. Qianting Zhang and colleagues from the Feng Lab and collaborative laboratories. This study describes a novel mechanism underlying TGF-β resistance in ALK-positive tumors.
Loss of TGF-β tumor suppressive response is a hallmark of human cancers. As a central player in TGF-β signal transduction, SMAD4/DPC4 is frequently mutated or deleted in gastrointestinal and pancreatic cancer. However, such genetic alterations are rare in most cancer types and the underlying mechanisms for TGF-β resistance remain largely unclear.
In the current study, Zhang and colleagues have elucidated a molecular mechanism for TGF-β resistance in ALK-positive tumors. Abnormal activation of ALK occurs in numerous cancers, including lymphoma, lung cancer, neuroblastoma, glioblastoma multiform and inflammatory breast cancer. Zhang and colleagues demonstrate that, in ALK-positive tumors, SMAD4 is highly phosphorylated. Molecular analyses show that ALK directly phosphorylates SMAD4 at Tyr95. Phosphorylated SMAD4 is unable to bind to DNA and fails to elicit TGF-β gene responses and tumor suppressing responses. Chemical or genetic interference of the oncogenic ALK restores TGF-β responses in ALK-positive tumor cells. These findings reveal, for the first time, that SMAD4 is tyrosine-phosphorylated by an oncogenic tyrosine kinase during tumorigenesis. This suggests a novel mechanism by which SMAD4 is inactivated in cancers and provides guidance in targeted therapies in ALK-positive cancers.
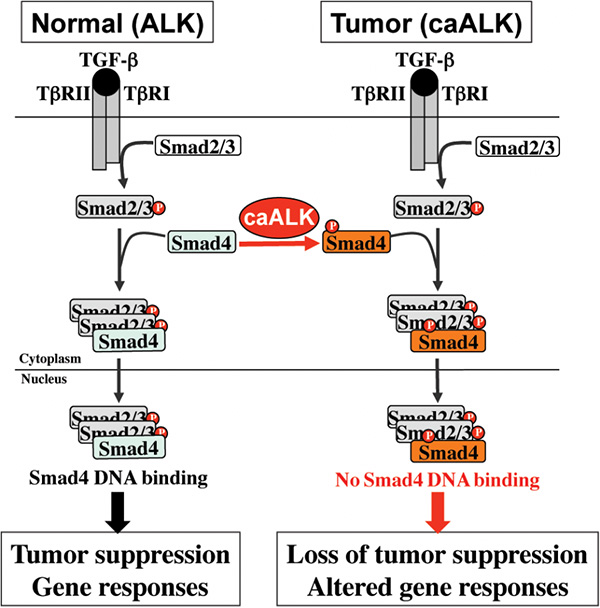
Cartoon: A working model for the role of tyrosine phosphorylation of SMAD4 on TGF-β signaling. Under normal physiological conditions, the SMAD complex transduces TGF-β tumor suppressing signals. In tumors expressing constitutively active ALK (caALK), SMAD4 is phosphorylated on tyrosine. Despite the presence of the SMAD complex, SMAD4 fails to bind to DNA and is unable to induce the expression of genes necessary for tumor suppression.
Links:https://www.nature.com/articles/s41556-018-0264-3



