Emotional reactions, including fear and stress, are considered normal psychological and physical reactions to positive or negative situations in our lives. However, frequent acute emotional reactions referred to as chronic stress are pathological conditions that increase the risk of depression and anxiety. In addition to causing anxiety behaviors, stress can lead to disorders of the immune, metabolic and cardiovascular systems. Some recent studies have also highlighted the physiological function of various immune molecules in the onset of anxiety-like behaviors. However, multiple questions regarding the effect of T cells on the onset of anxiety remain to be answered.
Professor Jin’s group published a research paper on Cell entitled “Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior” on October 31, 2019. This article revealed the critical link between a purine metabolic disorder in CD4+ T cells and stress-driven anxiety-like behavior.
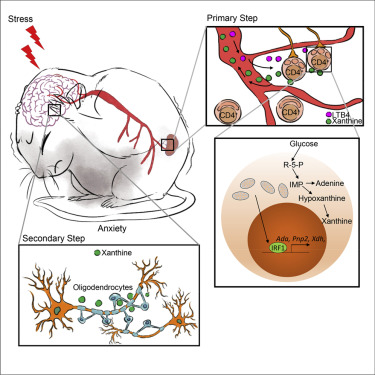
In this study, the authors used various animal behavioral assays including open-field test and elevated plus-maze to evaluate the anxious mood. Jin’s group found that the lack of CD4+ T cells protects mice from stress-induced anxiety-like behavior. Previous evidence has revealed that mitochondria morphology has been intimately linked to metabolic regulation in multiple cell types and tissues. Physical stress-induced leukotriene B4 triggers severe mitochondrial fission in CD4+ T cells, which further leads to a variety of behavioral abnormalities including anxiety, depression and social disorders. As the previous finding in Jin’s lab, IRF-1 was also accumulated in CD4+ T cells with fragmented mitochondria. Thus, the accumulated IRF-1 in these CD4+ T cells was significantly enriched at certain binding sites in the promoters of Ada, Pnp2 and Xdh, which further promotes the de novo synthesis of purine. As a result, most of the purines and their derivatives including adenine, hypoxanthine and xanthine were much times more abundant in anxious mice than in their WT littermates. Consistently, the author also found the serum xanthine was significantly higher in patients with anxiety, suggested that a similar physiological process has taken place in humans. A number of studies have suggested that the amygdala plays a critical role in generating fear and persistent anxiety. Metabolomic profiles and single-cell transcriptome reveals that CD4+ T cell-derived xanthine acts on oligodendrocytes in the left amygdala via adenosine receptor A1. Furthermore, the hyperactivity and hyperproliferation of oligodendrocytes caused more c-FOS expression on the neurons, directly promotes anxiety-like behavior.
In summary, the authors establish peripheral CD4+ T cells as pivotal mediators of stress-induced mood disorders. Most of the current therapeutic drugs for anxiety or depression are accompanied by many side effects. Therefore, it is of great significance to understand the pathogenesis of mood disorders to develop nontraditional therapeutic drugs. These results provide insights into the physiological function of adaptive immunity in neurodevelopment and neuropsychiatric disorders. These findings have profound implications for developing a valuable therapeutic approach for various psychiatric and metabolic diseases.
Ph.D. students Ke-qi Fan and Dr. Yi-yuan Li in Prof. Jin’s group are co-first authors. Professor Jin Jin and Professor Ren-jie Chai are co-corresponding authors of this article. This study was supported by the National Key R&D Program of China, Excellent Young Scientist Fund of NSFC, Zhejiang Provincial Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Science and the National Natural Science Foundation of China.
原文链接:https://www.sciencedirect.com/science/article/pii/S0092867419311171



