On July 1st, 2020, Dr. Junfang Ji's group in Life Sciences Institute of Zhejiang University published a research article in Hepatology, entitled “MiR-125b Loss Activated HIF1α/pAKT Loop, Leading to Trans-Arterial Chemoembolization Resistance in Hepatocellular Carcinoma”. It elucidated the role of miR-125b-mediated HIF1α signaling pathway in the transcatheter arterial chemoembolization (TACE) therapy resistance of hepatocellular carcinoma (HCC) patients and provided the potential potent biomarkers for assisting HCC patients to choose TACE therapy.
HCC, accounting for 80% of primary liver cancer, is the second leading cause of cancer death in men worldwide. More than half of new HCC cases and HCC-related death are in China annually, which poses a great threat to public health. According to AASLD and EASL HCC guidelines, TACE is the first-line therapy for intermediate stage (BCLC-B stage) HCC cases. Meanwhile, TACE is also used as an adjuvant therapy for early stage HCC cases after surgical tumor resection to reduce HCC recurrence in many Asian countries. Nevertheless, not all patients exhibit a survival benefit from adjuvant TACE. Adjuvant TACE is not widely accepted in Western countries either. In addition, there is no viable approach to identify HCC cases benefiting from TACE prior to therapy.
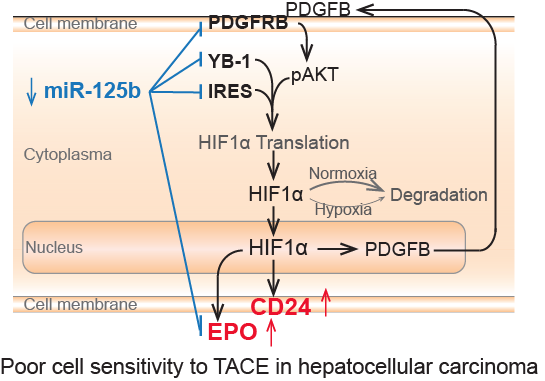
The authors employed four independent HCC cohorts with 680 patients and performed miRNA transcriptome analysis, molecular mechanism studies, as well as biomarker validations in clinical specimens. It discovered that miR-125b significantly inhibited HIF1α pathway activation, through which miR-125b regulated HCC cell sensitivity in response to TACE in both HCC cells and HCC patients.
Mechanistically, miR-125a attenuated HIF1α translation through directly binding onto internal ribosome entry site (IRES) of HIF1a mRNA, targeting YB1, and targeting PDGFβ receptor to restrain an autocrine HIF1α/PDGFβ/pAKT/HIF1α loop.
This study also revealed that hypoxia signaling and CD24-positive hepatic cancer stem cells rendered HCC cell resistance to TACE, which might potentially serve as molecular targets to improve TACE therapeutic efficacy. Meanwhile, the expression level of miR-125b and CD24 in HCC and EPO in serum of HCC cases predict clinical outcome of HCC patients with TACE therapy, which hold the potential to assist in identifying HCC cases benefiting from TACE prior to therapy.
Dr. Xiyang Wei from Junfang Ji’s group in Life Sciences Institute of Zhejiang University, and Prof. Lei Zhao from Shandong Cancer Hospital are co-first authors. Prof. Junfang Ji is the corresponding author. This work was supported by National Key R&D Program of China (2018YFA0800504), Zhejiang Basic Public Welfare Research Program (LZ20H160003), National Natural Science Foundation of China (No. 81874054 and 81672905).
Link:https://aasldpubs.onlinelibrary.wiley.com/doi/abs/10.1002/hep.31448



