H3K36 methylation is one of the important histone modifications, of which H3K36me3 has been mostly studied. H3K36me3 functions in many cellular processes including transcription initiation, pre-mRNA splicing, inhibition of cryptic transcription, RNA m6A modification, DNA repair and so on [1]. H3K36me2, which is currently less studied, is enriched at both gene bodies and intergenic regions and usually coordinates with other histone modifications to regulate gene expression. Previous studies have found that NSD1-mediated H3K36me2 could inhibit the expansion of H3K27me3 across the whole genome [2], thereby indirectly regulating gene expression. In addition, NSD1 mutations or abnormal expressions are also associated with many tumor diseases, including neuroblastoma, pediatric acute myelogenous leukemia, Sotos syndrome and gliomas [3,4]. However, the detailed mechanism by which H3K36me2 regulates gene expression is still unclear.
On June 9th, 2021, Dong Fang’s research team published an online research paper entitled The H3K36me2 methyltransferase NSD1 modulates H3K27ac at active enhancers to safeguard gene expression in Nucleic Acids Research, reporting that H3K27ac increases correlatively with the decrease of H3K36me2 at active enhancers in NSD1 knockout mouse embryonic stem cells (mESCs), which directly leads to the upregulation of mesoderm differentiation genes. At the same time, they also proposed that NSD1 can recruit the H3K27ac deacetylase HDAC1 to cooperatively regulate active enhancer activation.

This study found that in mESCs, knockout of NSD1 resulted in similar number of up-regulated genes and down-regulated genes. The down-regulated gene expression was mainly related to the gain of H3K27me3, while the up-regulated gene expression was mainly related to increase of H3K27ac at active enhancers. These up-regulated genes were mainly involved in the development of mESCs to mesoderm cells. NSD1 recruited HDAC1 and reduced H3K27ac signal at active enhancers, preventing the continuous activation of downstream gene caused by over-acetylation of active enhancers (Figure 1).
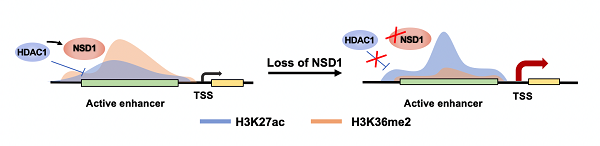
Figure 1. NSD1 recruits HDAC1 to coordinately regulate active enhancer-associated gene expression
NSD1 misregulation happens in various diseases which are difficult to treat and the development of specific inhibitors for H3K36me2 methyltransferases are difficult [5]. This study provides a new druggable target, HDAC1, for diseases caused by mutations or abnormal expressions of NSD1.
Dong Fang is the corresponding author of this article. Yuan Fang, Yin Tang, Yanjun Zhang that are all from Life Sciences Institute, Zhejiang University and Yixin Pan, who is from Zhejiang University School of Medicine, are the co-first authors. This work is supported and cooperated by the research group of Professor Ying Yuan, Zhejiang University School of Medicine. This work is also supported by the National Natural Science Foundation of China.
References
1. Huang, C. and Zhu, B. (2018) Roles of H3K36-specific histone methyltransferases in transcription: antagonizing silencing and safeguarding transcription fidelity. Biophys Rep, 4, 170-177.
2. Yuan, W., Xu, M., Huang, C., Liu, N., Chen, S. and Zhu, B. (2011) H3K36 methylation antagonizes PRC2-mediated H3K27 methylation. J Biol Chem, 286, 7983-7989.



