The human genome faces constant genotoxic insults that cause DNA damage and genomic instability. Upon sensing the DNA damage, PARP-1, as a founding member of the PARP family, is rapidly activated at the sites of DNA lesions and becomes auto-PARylated; this auto-PARylation event, in turn, creates a scaffold to recruit various downstream DDR factors to the damaged loci for DNA repair. However, due to the fast dynamics and lability of the PAR, the mechanism by which PARP1 initiates DDR, especially in the context of regulating transcriptional response, remains elusive.
Scientists from the labs of Life Sciences Institute member Huasong Lu, and Qiang Zhou, professor of biochemistry, biophysics and structural biology at UC Berkeley, published a research article entitled “Poly (ADP-ribosylation) of P-TEFb by PARP1 disrupts phase separation to inhibit global transcription upon DNA damage” in Nature Cell Biology on April 7, 2022. This new study suggests that the PARP1-P-TEFb signaling axis plays a protective role in transcription quality control and genomic stability maintenance when cells are exposed to DNA damage stress.
Previous work in Lu's lab has focused on transcriptional elongation condensates, unique membrane-less organelles that compartmentalize RNA polymerase II and various transcription elongation factors to enable robust transcriptional activation. In this work, researchers identified the transcriptionally engaged P-TEFb, a positive transcription elongation factor complex composed of CycT1 and CDK9, as a novel PAR-PARP1 interacting partner that orchestrates the DNA damage response. By leveraging the power of combined biochemical and cell biology approaches, the team revealed that PAR-mediated interactions between PARP1 and CycT1 are responsible for the subsequent PARylation of CycT1 in an HRD-dependent manner. This modification on CycT1 prevents it from undergoing liquid–liquid phase separation that is required for CDK9 to hyperphosphorylate Pol II and to stimulate elongation. Finally, the researchers found that the poly(ADP-ribosylation) of CycT1 promotes DNA repair and cell survival upon genotoxic stress. In summary, findings from this work provide some insight into the understanding of dynamic regulatory mechanism underlying biomacromolecule condensation and facilitate the identification of novel targets for developing effective and synergistic anticancer therapeutics.
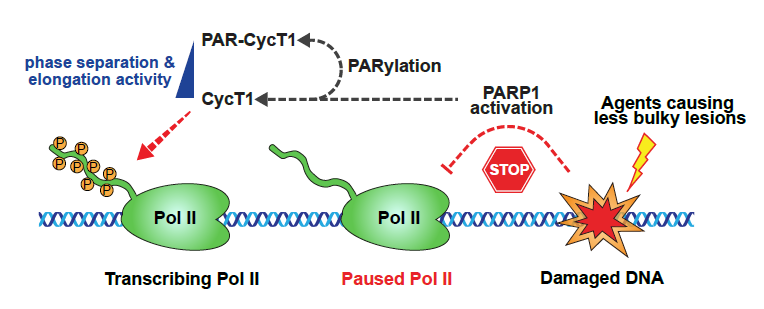
Figure: PARP1 PARylates CycT1 to promote transcriptional silencing upon DNA damage
Graduate students Huanyi Fu and Zixuan Jia from Lu’s lab and Dr. Rongdiao Liu from Xiamen University are co-first authors in this study. Prof. Huasong Lu, Xiang Gao and Qiang Zhou are the co-corresponding authors. This work was supported by the National Natural Science Foundation of China, Zhejiang Provincial Natural Science Foundation of China, and the institutional startup funds from LSI.