June 17th, 2022, Dr. Bin Zhao’s group published a research article entitled FUNDC2 promotes live tumorigenesis by inhibiting MFN1 mediated mitochondrial fusion on Nature Communications. Dr. Bin Zhao is the corresponding author, and Dr. Shuaifeng Li and graduate student Shixun Han are co-first authors. Drs. Cunqi Ye, Xin-Hua Feng, and Tingbo Liang’s groups also made important contributions to this work.
Primary liver cancer is the fourth leading cause of cancer death worldwide, and the current treatment is very limited. Hepatocellular carcinoma (HCC, accounting for 75%-85%) is the most common type of liver cancer. As the cellular power house, dysregulation of mitochondria in cancer was observed for a long time. However, the mechanism of mitochondria fragmentation in cancer was not yet clear.
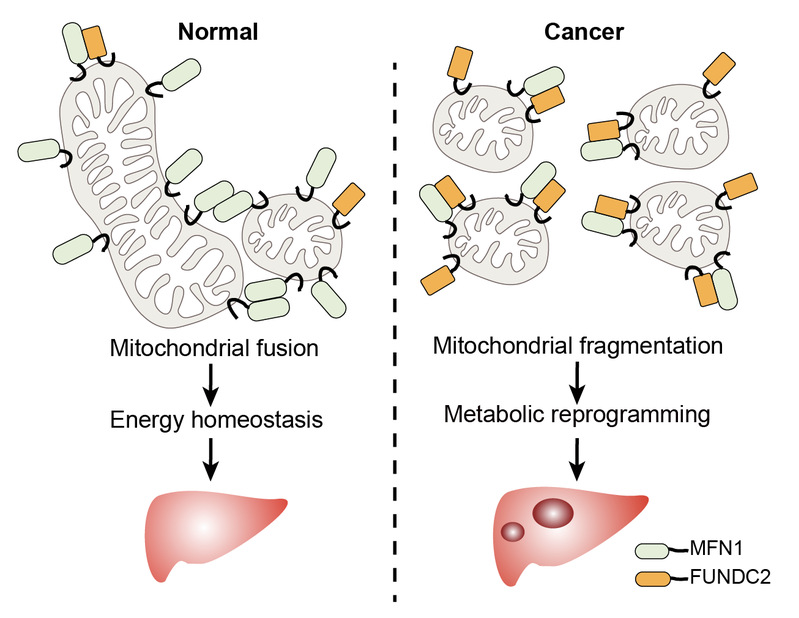
This study systematically screened dysregulated mitochondrial proteins in HCC, and identified FUNDC2 as an elevated protein associated with poor prognosis. Furthermore, elevated expression of FUNDC2 was also found in MYC+RAS mouse liver tumors. Knockdown of FUNDC2 by multiplexed genome editing significantly inhibited tumorigenesis induced by MYC+RAS. In addition, knockdown of FUNDC2 rescued mitochondrial fragmentation in tumors, and reduced mitochondrial respiration.Targeted metabolomics, lipidomics, glucose flux and palmitic acid flux assays revealed that knockdown of FUNDC2 significantly inhibited glycolysis, TCA cycle and fatty acids β-oxidation. Knockdown of FUNDC2 also led to accumulation of triglycerides and greatly reduced the level of phospholipids. Mechanistic studies found that FUNDC2 inhibits MFN1 activity by binding to the GTPase domain, thereby inhibiting mitochondrial fusion.
In conclusion, this work demonstrated MFN1 inhibition by FUNDC2 as a mechanism of mitochondrial fragmentation, which contributes to tumorigenesis of HCC. These results also suggest FUNDC2 as a potential therapeutic target of HCC.



