Due to the increased usage and reduced cost of high-throughput sequencing, a variety of sequencing technologies are extensively developed and applied in life science research, especially for analyzing genome-wide events. The traditional method for high-throughput library preparation involves fragmentation of DNA, which is done by mechanical sonication or enzyme digestion, and the ligation of designed DNA primers to the fragmented DNA [1]. Then the DNA is ready to be amplified by PCR to construct the sequencing library.
Tn5 transposons found in E. coli have been widely used to tagment double-strand DNA and DNA/RNA hybrids [2]. Transposons can fragment and insert transposon cargo primers into the targeted DNA, thus producing DNA fragments that can be directly amplified by PCR for sequencing [3,4]. Compared with traditional methods, it combines the process of DNA fragmentation and ligation of designed DNA primers, resulting in reduced library preparation time and increased sensitivity. Therefore, Tn5 has been widely applied to high-throughput sequencing.
On April 4th, 2023, Dr. Dong Fang’s laboratory published a paper entitled Tn5 tagments and transports oligos to single-strand DNA for strand-specific RNA sequencing in Genome Research, proposing that Tn5 tagments single-strand DNA and can be used for strand-specific single-strand DNA sequencing.
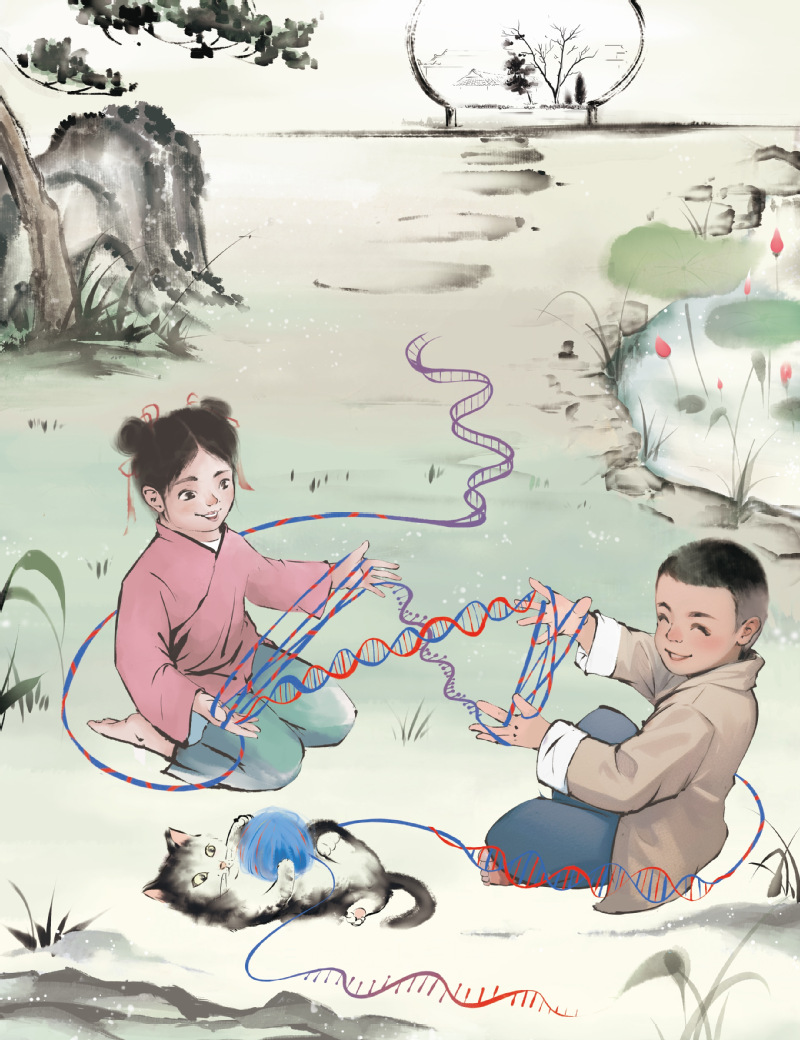
Fig 1. Tn5 tagments different kinds of nucleic acids. Two kits are playing cat's cradle on the playground just like two Tn5 transposases act at different types of nucleic acids. Two kids represent two Tn5 transposases; the strings used by the two kids represent double-strand DNA, single-strand DNA, and DNA/RNA hybrid. The substrates used by Tn5 transposase are very diverse.
To explore how Tn5 works on different nuclear acid substrates, researchers analyzed double-strand DNA, single-strand DNA, RNA strand, and DNA/RNA hybrid strand as target DNA, and found that Tn5 tagments single-strand DNA. Mechanistically, Tn5 inserted transposon cargo DNA into the 5' end of single-strand DNA. Based on this characteristic, researchers developed a new high-throughput sequencing method for sequencing single-strand DNA. This method is called TABLE-seq (a tagmentation-based and ligation-enabled single-strand DNA sequencing method).
In TABLE-seq, a special splint DNA adaptor was designed to ligate to the 3' end of tagmented single-strand DNA. This specially designed adaptor was synthesized by annealing two oligos. One oligo was phosphorylated at the 5’ end to be ligated to single-strand DNA. The other oligo contained a protruding end of 6 random DNA base pairs, which increased the efficiency of complementary pairing to the single-strand DNA. In addition, an inverted dT was added to the 3’ end to prevent mis-ligation and reduce the extension of the random hexamer-containing stand. The Tn5 fragmented single-strand DNA which was ligated with this adaptor could be amplified by PCR to obtain a strand-specific DNA library for high-throughput sequencing.
Researchers then applied this TABLE-seq method to strand-specific RNA sequencing, using the reverse transcribed single-strand cDNA as the substrates. Compared with the traditional dUTP-based method, this new method doesn’t require UDGase to digest the dUTP-containing second strand cDNA. Moreover, this new method detects more genes, has a higher strand-specificity, and shows more evenly distributed across genes.
This study discovered the activity of Tn5 to use single-strand DNA as the substrate, resulting in the development of a new strand-specific single-strand DNA sequencing method. This research expanded the potential of Tn5 in high-throughput sequencing.
Yanjun Zhang and Yin Tang, Ph.D. students from Life Sciences Institute, Zhejiang University, are the co-first authors. Dr. Dong Fang is the corresponding author.
References
[1] Oyola SO, Otto TD, Gu Y, Maslen G, Manske M, Campino S, Turner DJ, Macinnis B, Kwiatkowski DP, Swerdlow HP et al. 2012. Optimizing Illumina next-generation sequencing library preparation for extremely AT-biased genomes. BMC Genomics 13: 1.
[2] Sun Z, Tang Y, Zhang Y, Fang Y, Jia J, Zeng W, Fang D. 2021b. Joint single-cell multiomic analysis in Wnt3a induced asymmetric stem cell division. Nature Communications 12: 5941.
[3] Adey A, Morrison HG, Asan, Xun X, Kitzman JO, Turner EH, Stackhouse B, MacKenzie AP, Caruccio NC, Zhang X et al. 2010. Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition. Genome Biol 11: R119.
[4] Hennig BP, Velten L, Racke I, Tu CS, Thoms M, Rybin V, Besir H, Remans K, Steinmetz LM. 2018. Large-Scale Low-Cost NGS Library Preparation Using a Robust Tn5 Purification and Tagmentation Protocol. G3 (Bethesda) 8: 79-89.
Link: https://genome.cshlp.org/content/33/3/412.full