Recently, the research group led by Professor Shixian Lin from Life Sciences Institute, in collaboration with other teams, published a comprehensive review paper titled The Biosynthesis and Applications of Protein Lipidation in Chemical Reviews. This paper provides an in-depth review of the advancements in the biosynthesis of protein lipidation and its applications in biomedical research. The first authors of the paper include Dr. Wenlong Ding, Dr. Wenyuan Xu, joint graduate student Jiayu Gu, as well as Jing Wu and Yiwen Huang from Huazhong Agricultural University. Professor Shixian Lin and Professor Shuai Zhang from Huazhong Agricultural University are the corresponding authors of the paper. Chemical Reviews, founded in 1924 by the American Chemical Society (ACS), is a highly respected journal that publishes comprehensive, authoritative, and critical analyses of cutting-edge research across all fields of chemistry, including biochemistry. It serves as a valuable resource for researchers by offering high-quality, integrated reference materials. The journal also regularly publishes thematic issues, which focus on emerging areas or interdisciplinary research. Notably, this review will be collected in the recently published thematic issue on Synthetic Biology.
Protein lipidation involves the covalent attachment of hydrophobic lipid molecules to specific sites on proteins, modulating protein-membrane and protein-protein interactions. Lipidation plays a crucial role in nearly all membrane-associated biological processes. Although thousands of proteins in cells undergo lipidation, the functions of lipidation on many proteins remain unclear due to the modification's highly variable, reversible nature and its interactions with other post-translational modifications. Additionally, the peptide or protein drugs with lipidation modifications significantly extend their serum half-life by binding to serum albumin. Lipidation is a clinically proven strategy for the development of long-acting drugs and has been successfully applied in the development of several blockbuster marketed drugs, such as semaglutide and tirzepatide. Therefore, exploring the synthetic biology of protein lipidation not only aids in understanding its molecular mechanisms and biological functions but also offers valuable strategies for the design and development of novel lipidated therapeutics.
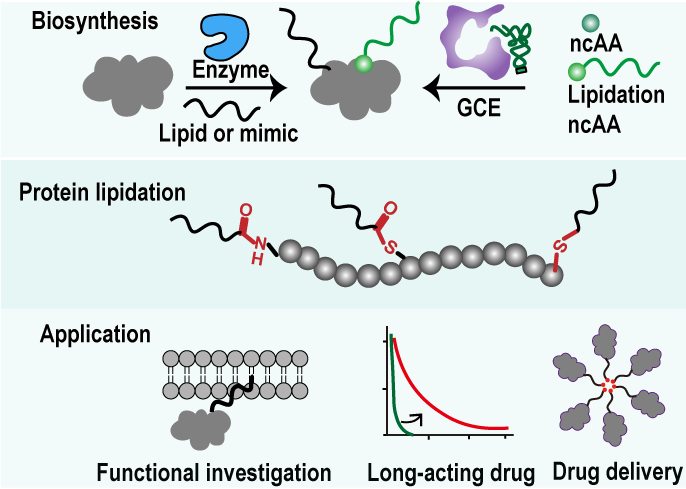
Figure 1: Summary schematic diagram of synthetic biology research on protein lipidation.
In this review, the authors first introduce the enzymatic pathways involved in protein lipidation modifications in mammalian cells, summarizing the types and mechanisms of natural lipid modifications. The paper then reviews the strategies for the biosynthesis of lipidated proteins by engineering natural modification enzymes and substrates, highlighting their applications in mechanism research. Additionally, the review presents the strategies for site-specific lipidation of proteins mediated by lipid transferases and ligases, focusing on in vitro biosynthesis techniques and their applications. The paper also examines approaches for achieving site-specific lipidation in E. coli and mammalian cells, summarizing key applications of these strategies in studying lipid modifications' biological functions and drug development. Moreover, the paper evaluates methods for using small-molecule probes to regulate the biosynthesis of protein lipidation in living cells. Finally, the review discusses the challenges and future directions in the field of synthetic biology for protein lipidation modifications.
This research was supported by grants from the National Natural Science Foundation of China and the Ministry of Science and Technology.



