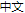Research interests:
We are broadly interested in the roles of chemical modifications in determining protein function, localization and stability. Our main focus is to uncover the regulatory mechanisms of the 26S proteasome under various pathophysiological conditions and elucidate their biological meanings in development, diseases and aging.
 Protein synthesis and degradation are essential and equally important for the maintenance of proteostasis, the very basis of life. From yeast to human, the vast majority of cellular proteins in eukaryotes are degraded by the ubiquitin-proteasome system (UPS). Sitting at the core of this system, the proteasome is indispensable for almost every biological process in the cell, from mitosis to DNA repair, from gene expression to disposal of cellular trash, from signal transduction to cell death. It is safe to say that any biological activity involving proteins relies on the proper functioning of the proteasome.
Protein synthesis and degradation are essential and equally important for the maintenance of proteostasis, the very basis of life. From yeast to human, the vast majority of cellular proteins in eukaryotes are degraded by the ubiquitin-proteasome system (UPS). Sitting at the core of this system, the proteasome is indispensable for almost every biological process in the cell, from mitosis to DNA repair, from gene expression to disposal of cellular trash, from signal transduction to cell death. It is safe to say that any biological activity involving proteins relies on the proper functioning of the proteasome.
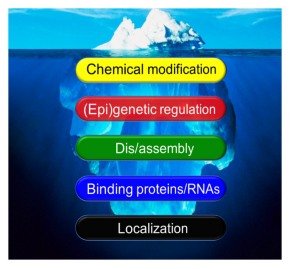 Despite its undeniable importance, the proteasome has long been regarded no more than a “cellular trashcan” that is constitutively active, non-selective and always on call. This leads to neglect of a vitally important question: how is the proteasome itself regulated? In fact, it has become increasingly clear that the proteasome changes its properties during cell proliferation, stem cell differentiation, signal transduction, immune responses, neural transmission, stress responses, etc. One the other hand, dysregulation of the proteasome has been implicated in a variety of human diseases including cancer, autoinflammation, neurodegeneration and cardiovascular disorders, and it is also one of the hallmarks of aging. By and large, our knowledge about proteasome regulation is just the tip of the iceberg.
Despite its undeniable importance, the proteasome has long been regarded no more than a “cellular trashcan” that is constitutively active, non-selective and always on call. This leads to neglect of a vitally important question: how is the proteasome itself regulated? In fact, it has become increasingly clear that the proteasome changes its properties during cell proliferation, stem cell differentiation, signal transduction, immune responses, neural transmission, stress responses, etc. One the other hand, dysregulation of the proteasome has been implicated in a variety of human diseases including cancer, autoinflammation, neurodegeneration and cardiovascular disorders, and it is also one of the hallmarks of aging. By and large, our knowledge about proteasome regulation is just the tip of the iceberg.
As one of the few labs in China specialized in proteasome research, our goal is to understand the multi-level regulations of the proteasome using diverse advanced approaches and through extensive collaborations.
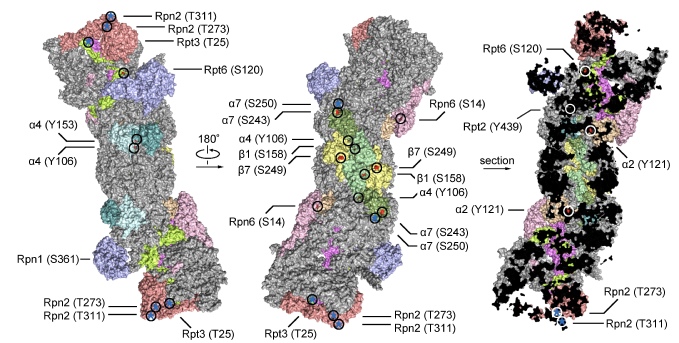
Phosphorylation is one of the most common modifications of the proteasome, through which the proteasome is integrated into the vast signaling network of the cell. We have made significant contributions to understanding proteasome phospho-regulation and accumulated much experience in this field. Shown below are a small fraction of proteasome phosphosites that are known or expected to have functional importance. It is noteworthy that phosphosites on the human 26S proteasome amount to more than 450, while over 95% of them remain uncharacterized.
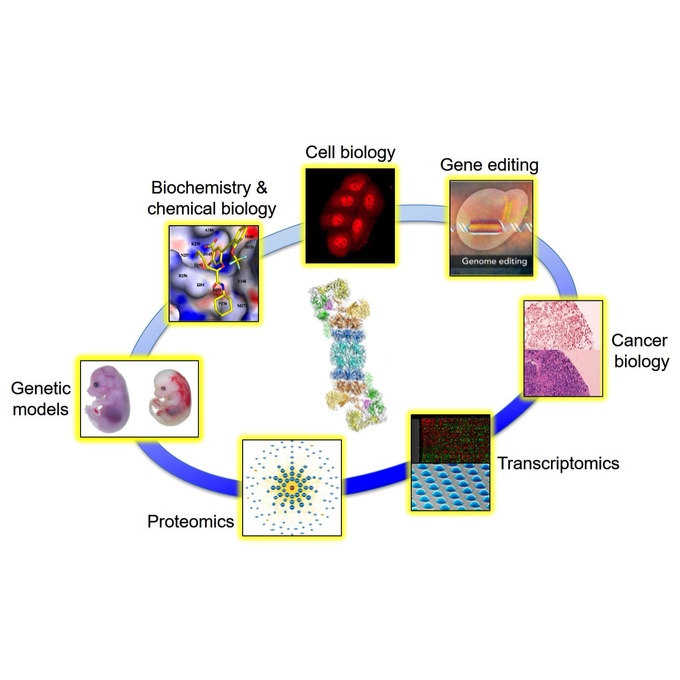
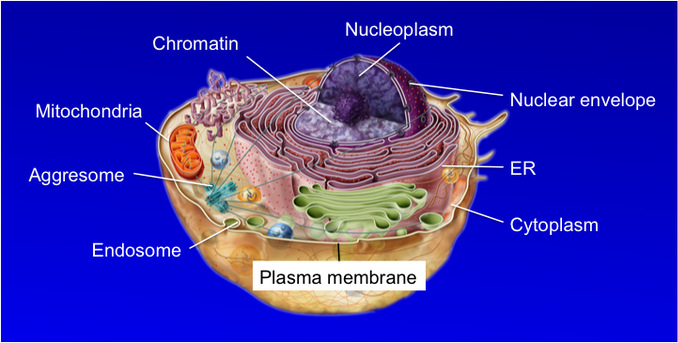
Another common misconception about the proteasome is that it only resides and functions in the cytoplasm. The truth is that the proteasome is found almost everywhere inside a cell, including in association with various membrane structures (see diagram below). How the proteasome localizes to these distinct parts of cell and the biological relevance of compartmentalized protein degradation are also intriguing questions currently under investigation in our lab.
In the past years, we have
· discovered the first proteasome-specific pS/pT phosphatase
· identified the first proteasome-regulating phospho-tyrosine phosphatase
· demonstrated for the first time that the proteasome itself undergoes dynamic phosphorylation during cell cycle, which in turn promotes cell cycle progression
· for the first time employed the genetic code expansion approach to provide the best biochemical explanation of how site-specific phosphorylation determines proteasome assembly
· revealed the first evidence of unique phospho-regulation of membrane-associated proteasomes and its role in controlling localized protein degradation
· made a series of proprietary antibodies toward specific proteasome modifications
· engineered several genetically modified mouse strains for proteasome research
.
.
.
Based on established cell biology and biochemical systems, we have incorporated a repertoire of advanced approaches including multi-omics, structural biology, chemical biology, gene editing, high-end imaging, cancer biology, neurobiology and animal behavioral studies in order to gain a comprehensive understanding of proteasome regulation and protein degradation. We also look forward to translating our discoveries in basic research to technologies and products of clinical and commercial values.
|