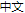Research Interests
We are interested in the molecular and cellular origin of organ size control in animals and the related abnormalities that leads to cancer. Currently, we are working on the following directions:
1.Molecular mechanisms of the Hippo pathway in organ size control
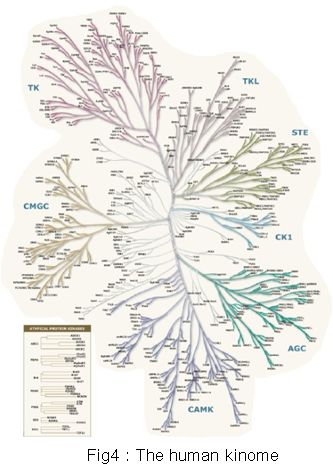
A relatively constant organ size is one of the most remarkable features of multi-cellular organisms. For instance, the mouse liver can recover to the original size after partial hepatectomy. However, little is known about the underlying molecular mechanisms. Recent research demonstrated a crucial role of the Hippo pathway in organ size control, cancer development, tissue regeneration and stem-cell differentiation. For example, tissue-specific ablation or overexpression of the Hippo pathway proteins leads to dramatic alteration of liver size in mice. At the center stage of the Hippo pathway is a kinase cascade consists of Mst1/2 and Lats1/2 kinases. Lats1/2 activated by Mst1/2 could phosphorylate and inhibit a transcription co-activator YAP and its paralog TAZ, thus impacts downstream target gene expression which functions in multiple aspects of cell physiology such as proliferation, apoptosis and differentiation (Fig.1). Latest research identified cell adhesion status, cell-cell contact, cytoskeleton, and more recently GPCR signaling as upstream stimuli of the Hippo pathway. However, specific molecular mechanisms remain elusive. Our lab seeks these molecular mechanisms through an integrative approach. Answering these questions would provide invaluable insights into the mystery of organ size control and pave a way for future application in anti-cancer therapy and regenerative medicine.
2.Deregulation of cell signaling in cancer
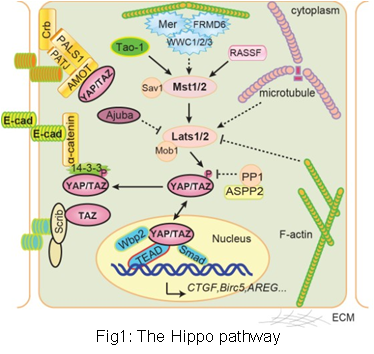
Cancer is one of the most notorious obstacles to human health. One of the key features of cancer is that the proliferation, apoptosis, differentiation, migration and metabolism of cancer cells is uncoupled from the surrounding normal tissue and matrix. Furthermore, it is more and more appreciated that the interplay between stromal and cancer cells play a key role in shaping the distinctive behavior of cancer cells. Both normal cells and cancer cells communicate with their environment through signal transduction pathways. Elucidate the signaling mechanism of cell-environment communication is thus the key to understand and cure cancer. Accumulating evidence suggests that deregulation of the Hippo pathway plays a key role in tumorigenesis and cancer progression. For example, we found elevated YAP protein level and nuclear localization in human cancers (Fig.2) and down-regulation of tumor suppressor Lats1/2 in metastatic prostate cancer. Furthermore, it has been reported that TAZ, the YAP paralog, plays an important role in the self-renewal of breast cancer stem cells. Interestingly, it was recently demonstrated that the Hippo pathway is a key mediator of both physical and chemical signals of the cell microenvironment. Thus understanding the Hippo pathway is a key to solve the deregulated communication between cancer cells and the organism. Our lab uses various models to mimic cancerous transformation process (Fig.3) in order to study the deregulation and functions of the Hippo pathway in cancer development.

3.Protein kinases and signal transduction
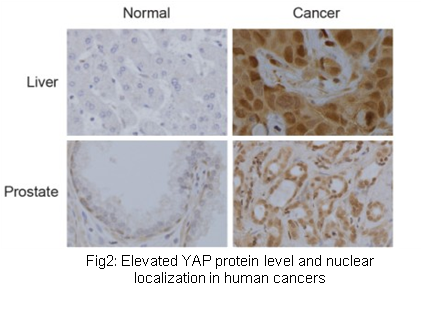
Protein phosphorylation catalyzed by protein kinases regulates protein functions through various mechanisms. It is also a pivotal biochemical mechanism establishing the cell signaling network. Mutation, translocation, gene amplification, and altered expression of protein kinases are key molecular mechanisms leading to cancer. Thus protein kinases are also main targets of current targeted anticancer drugs. Therefore, it is highly important to systematically study the function and regulatory mechanisms of protein kinases in physiological and pathological conditions at the genomic level. Our laboratory is experienced in molecular characterization of protein kinases. We are establishing a platform for functional screen of the human kinome (Fig.4). On the basis, we mainly study kinases regulating the Hippo signaling pathway and other signal pathways in human cancer.